CHEMICAL ELEMENT :Potassium
Overview
Potassium is one of the alkali metals. The alkali metals are the elements that make up Group 1 (IA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. The alkali metals also include lithium, sodium, rubidium, cesium, and francium. They are among the most active metals.
Potassium is so active that it never occurs free in nature. It always occurs in compounds, combined with other elements. It was first prepared in pure form in 1807 by English chemist Sir Humphry Davy (1778-1829). Davy used a new method of isolating elements that he had invented, electrolysis. In electrolysis, an electric current is passed through a molten (melted) compound. The electrical current breaks the compound into its elements. (See sidebar on Davy in the calcium entry in Volume 1.)
There are very few uses for potassium as a pure element. However, compounds of potassium have many important applications, the most important of which is as a fertilizer.
SYMBOL
K
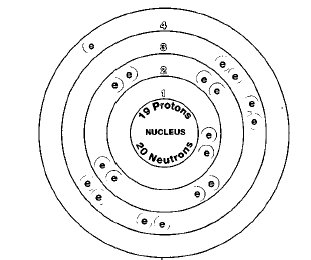
ATOMIC NUMBER
19
ATOMIC MASS
39.0983
FAMILY
Group 1 (IA)
Alkali metal
PRONUNCIATION
poe-TAS-see-um
Discovery and naming
Early humans were familiar with potash, a potassium compound that forms when wood burns. Wood ashes were washed with water to dissolve the potash. It was then recovered by evaporating the water. Potash was often called vegetable alkali. That name comes from the origin of the material ("vegetable" plants that contain wood) and the most important property of the material, alkali. The word alkali means a strong, harsh chemical that can be used for cleaning. Common household lye (such as Drano) is a typical alkali.
For many centuries, people had trouble telling "vegetable alkali" and "mineral alkali" apart. The two materials looked and acted very much alike. For example, they could both be used as cleaning materials. The main difference between them was the source from which they came. It was not until the eighteenth century that chemists understood the difference between potash (vegetable alkali) and soda ash (mineral alkali).
By the late 1700s, chemists were reasonably sure that both potash and soda ash contained elements they had never seen. They tried to think of ways to break these compounds down into their elements. The first method that Davy tried was to pass an electric current through a water solution of one compound or the other. But no new element was ever formed. What Davy did not know was how active the elements potassium and sodium are. Both elements are freed when an electric current is passed through a water solution of potash or soda ash. But as soon as the element is formed, it reacts immediately with the water. The free element can never be recovered by this method.
Then Davy thought of another way to separate potash and soda ash into their elements. He decided to use no water in his experiment. Instead, he melted a sample of potash and a sample of soda ash. Then he passed an electric current through the molten (melted) substances. He was amazed to see a tiny liquid droplet of metal formed in each case. The droplet was the first piece of potassium and sodium ever to be seen by a human.
Davy had his first success with potassium using this approach on October 6, 1807. A few days later he repeated his experiment with soda ash and produced pure sodium metal. Davy named these two elements after their much older names: potassium for "potash" and sodium for "soda ash."
Physical properties
Potassium is a soft, silvery-white metal with a melting point of 63°C (145°F) and a boiling point of 770°C (1,420°F). Its density is 0.862 grams per cubic centimeter, less than that of water (1.00 grams per cubic centimeter). That means that potassium metal can float on water. Chemically, though, that's not a good idea (see "Chemical properties" below).
The melting point of potassium is very low for a metal. It will melt over the flame of a candle flame.
Chemical properties
Like the other alkali metals, potassium is very active. It reacts with water violently and gives off hydrogen gas:
So much heat is produced in this reaction that the hydrogen gas actually catches fire and may explode. Floating potassium metal on the surface of water is not a good idea! In that instance, the potassium would skip along the surface of the water. The skipping is caused by hydrogen gas produced in the reaction pushing the metal around. The potassium would soon catch fire, burn, and, perhaps, explode.
Potassium reacts readily with all acids and with all non-metals, such as sulfur, chlorine, fluorine, phosphorus, and nitrogen.
Occurrence in nature
Potassium is the eighth most abundant element in the Earth's crust. Its abundance is estimated to be about 2.0 to 2.5 percent. It is just slightly less abundant than its alkali cousin, sodium.
Potassium is less dense than water, so it can float on water. However, chemically, potassium reacts with water violently. It will give off hydrogen and eventually catch fire.
Potassium occurs widely in many different minerals. Some of the most important of these are sylvite, or potassium chloride (KCl); sylvinite, or sodium potassium chloride (NaCl KCl); carnallite, or potassium magnesium chloride (KCl MgCl 2 ); langbeinite, or potassium magnesium sulfate (K 2 SO 4 ○ 2MgSO 4 ); and polyhalite, or calcium magnesium potassium sulfate (2CaSO 4 ○ MgSO 4 ○ K 2 SO 4 ).
Isotopes
There are three naturally occurring isotopes of potassium, potassium-39, potassium-40, and potassium-41. Potassium-40 is radioactive. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
About ten artificial radioactive isotopes of potassium are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Artificially radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
Potassium-40 is of special interest to scientists. Potassium is widely distributed in nature in plants, animals, and rocks. That means that nearly everything on Earth contains at least a tiny amount of radioactive potassium-40. That includes the human body! About 0.012 percent of the potassium in the human body is radioactive potassium-40. However, that is not enough radiation to cause any harm.
Radioactive potassium-40 in rocks can be used to measure the age of objects. When the isotope gives off radiation, it breaks down to an isotope of argon:
A scientist can analyze a rock to see how much potassium-40 and how much argon-40 it contains. The older the rock, the more argon-40 and the less potassium-40 it contains. The younger the rock, the more potassium-40 and the less argon-40 it contains.
One might wonder why argon gas does not escape into the atmosphere. The answer is that argon gas is trapped within the solid rock. It is released only when the potassium-dating process is conducted.
Extraction
The word "potash" is still a widely used term for potassium compounds taken from the earth. But it no longer means potassium carbonate to most people. It can mean potassium sulfate (K 2 SO 4 ), potassium chloride (KCl), potassium nitrate (KNO 3 ), potassium hydroxide (KOH), or potassium oxide (K 2 O). People cling to "potash" because it is the term used in the manufacture of fertilizers. And fertilizers are far and away the most important use of potassium compounds today.
Chemistry and the environment
E nvironmentalists often say that everything in nature is related. Here is a good example of that principle:
Potash was a widely used material in Colonial America. People used the compound to make soap, glass, and dozens of other products. At the time, potash was easy to get. All one had to do was burn a tree and collect potash from its ashes.
The only problem was that a single tree does not produce much potash. To get all the potash a family might need, one might have to burn dozens or hundreds of trees. Colonists did not worry too much about this problem. America in the 1700s was covered with trees. Few people thought about or cared about "saving the environment." If they ran out of trees, they just moved farther west.
One can imagine what America would have looked like if Colonists continued this practice. Fortunately, they did not have to. In the 1780s, French chemist Nicolas Le Blanc (1742-1806) invented an inexpensive method for making soda ash. Le Blanc's method used salt, or sodium chloride (NaCl); limestone, or calcium carbonate (CaCO 3 ); and coal (pure carbon). These three materials are all common and inexpensive. The Le Blanc method of making soda ash is quick, easy, and cheap. Before long, soda ash had become one of the least expensive chemicals made artificially. In the United States, trees were no longer burned to get potash.
The most important source of potash in the United States is a mine near Carlsbad, New Mexico, that produces sylvinite (KCl NaCl). That mine produces about 85 percent of the potash mined in the United States. Potash is also produced from huge long-buried salt mine blocks formed when ancient seas evaporated (dried up). In Michigan, for example, potash is obtained

Potassium metal is produced by combining potassium chloride with sodium metal at high temperatures. But this method is of little interest because potassium metal has few uses.
Uses
Potassium metal is sometimes used as a heat exchange medium. A heat exchange medium is a material that picks up heat in one place and carries it to another place. Potassium metal is sometimes used as a heat exchange medium in nuclear power plants. There, heat is produced at the core, or center, of the reactor. Liquid potassium is sealed into pipes surrounding the core. As heat is given off, it is absorbed (taken up) by the potassium. The potassium is then forced through the pipes into a nearby room. In that room, the potassium pipes are wrapped around pipes filled with water. The heat in the potassium warms the water. Eventually the water gets hot enough to boil. It changes into steam and is used to operate devices that generate electricity.
Compounds
By far the most important compound of potassium is potassium chloride (usually referred to as "potash," of course!). At least 85 percent of that compound is used to make synthetic (artificial) fertilizers. In 1996, about 1.5 billion kilograms (3.4 billion pounds or 1.7 million tons) of potassium chloride was produced in the United States for use in fertilizers.
Many other potassium compounds are commercially important, although no use begins to compare with the amount of potash used for fertilizers. Some examples of other important potassium compounds are the following:
Potassium is one of the three primary nutrients, or macronutrients, required by plants.
potassium bicarbonate, or baking soda (KHCO 3 ): baking powders; antacid (for upset stomach); food additive; soft drinks; fire extinguishers
potassium bisulfite (KHSO 3 ): food preservative (but not in meats); bleaching of textiles and straw; wineand beer-making; tanning of leathers
potassium bitartrate, or cream of tartar (KHC 4 H 4 O 6 ): baking powder; "tinning" of metals; food additive
potassium bromide (KBr): photographic film; engraving
potassium carbonate, or potash (K 2 CO 3 ): specialized glasses and soaps; food additive
potassium chromate (K 2 CrO 4 ): dyes and stains (bright yellowish-red color); explosives and fireworks; safety matches; tanning of leather; fly paper
potassium fluorosilicate (K 2 SiF 6 ): specialized glasses, ceramics, and enamels; insecticide
potassium hydroxide, or caustic potash (KOH): paint remover; manufacture of specialized soaps; fuel cells and batteries; bleaching; food additive; herbicide
potassium nitrate, or nitre, or saltpeter (KNO 3 ): explosives, fireworks, matches, rocket fuel; manufacture of glass; curing of foods
potassium pyrophosphate, or tetrapotassium pyrophosphate, or TKPP (K 4 P 2 O 7 ): soaps and detergents Potassium bicarbonate is an additive in fire extinguishers.
Potassium bicarbonate is an additive in fire extinguishers.potassium sodium tartrate, or Rochelle salt (KNaC 4 H 4 O 6 ): baking powder; medicine; silvering of mirrors
Health effects
Potassium is essential to both plant and animal life. It is one of the three primary nutrients, or macronutrients, required by plants. Plants require relatively large amounts of potassium in order to grow and remain healthy.
Potassium plays a number of important roles in the human body also. It helps control the proper balance of fluids in cells and body fluids. It is involved in the transmission of chemical messages between nerve cells and in the contraction of muscles. Potassium also helps in the digestion of food and in the proper function of the eyes. In many of these reactions, potassium and sodium work together to keep these functions performing properly.
The average human who weighs 70 kilograms (150 pounds) has 140 grams (5 ounces) of potassium in his or her body. Normal daily intake of potassium is about 3.3 grams (0.1 ounce). Since potassium occurs in all plants, humans normally do not have any problems getting enough of the element in their daily diet.
